BOGGED DOWN AGAIN?
In GMP-compliant production in cleanrooms, the highest levels of accuracy, purity and safety count. But even today, many documentation processes still take place in paper form and are therefore prone to errors. More and more laboratories are recognizing the opportunities offered by paperless production.

WHAT REALLY MATTERS
What our customers in the life sciences industry expect from digital signage or paperless production in a manufacturing environment
- Reliable system for stable, GMP-certified operation of all operational processes
- Easy installation and operation
- Flexibility and quick adaptability
- Data security
- Increased efficiency of production processes
PEN AND PAPER WAS YESTERDAY.
LSR.SIGNAGE IS TODAY.
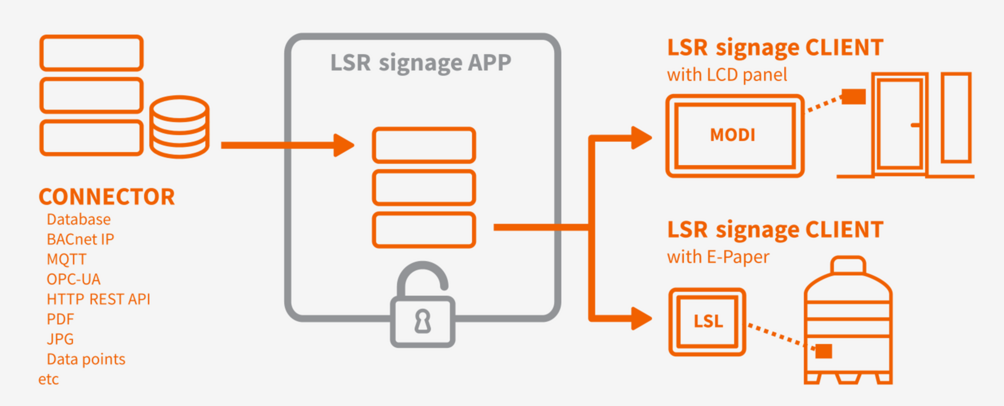
LSR.signage from Systec & Solutions is the app for digital signage and paperless production in the life science industry. The LSR.signage app uses data from your database. With this app, information you have defined is displayed directly on digital dashboards in front of your cleanrooms or directly on equipment. Status changes are visible in real time. This accurate communication increases your production safety and boosts your efficiency. Because there is no room for error in the cleanroom.
VALID DATA SOURCE
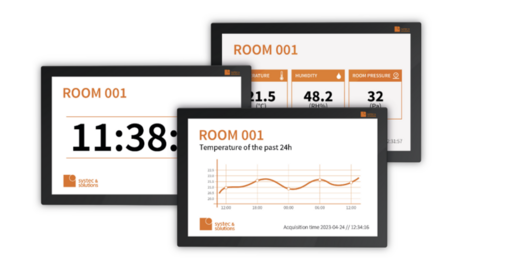
FLEXIBLE AND SCALABLE
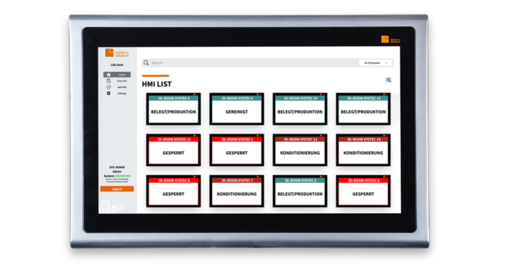
SAFETY MEETS DESIGN
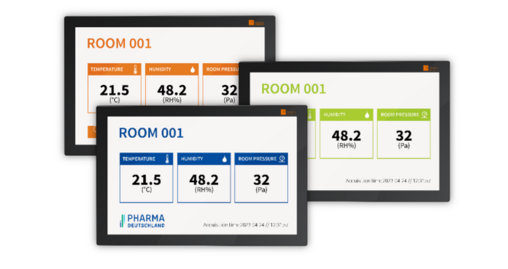
CAN ALSO BE INTEGRATED AT A LATER DATE

QUICK TO USE
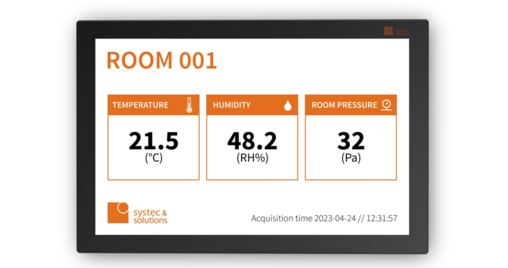
E-PAPER-BASED LABELS

EVERYTHING THAT COUNTS ON SITE:
LSR.SIGNAGE DISPLAYS IT.
All data from your database in real time in the right place:
Via MODI on your cleanrooms or LSL e-paper-based labels on your equipment.
Arrange a demo appointment. We'll talk about your requirements and show you how our dashboards and LSR.signage software can free your cleanrooms and equipment from paper.
“The implementation of LSR.signage was quite simple, which saved time and therefore costs. And the license model for the software is very fair, so we were able to take this step easily.”
Dennis Wild, Product Manager Digitalization Excella GmbH & Co. KG
DESIGN MEETS FUNCTION
This is the alternative to clipboards and pens: Clear screen design according to your specifications. Validated, always up-to-date and reliable data at a glance.

POWER
PoE (Power over Ethernet)
COMPATIBLE
with Linux, Windows 11, IGEL
CLEARLY
7“ or 10.1” LCD display, fanless with IP65
EASY
Simple flush mounting with Easy Click Magnetic Mount
CLEAN
Safe cleaning, IP65-compliant
USER-FRIENDLY
PCT-Multi-Touchscreen with Optical Bonding
RELIABLE
- High-end software for high-end hardware
- Cleanroom data displayed can be individually adapted to your needs in the admin area
- Timestamp with last update time
NEW: THE E-PAPER-BASED LSL

SAFETY
All important information now also available digitally on your equipment withE-Paper LSL
FLEXIBLE
Wireless plug-and-play functionality, approx. 5 year battery life
LIFE SCIENCE READY
WITH SYSTEC & SOLUTIONS
We specialize in solutions for the pharmaceutical industry. And are ready for your life science challenges. We support you from GMP-compliant planning to the implementation of HMI systems with LSR.signage, right through to validated data transport.
FAQ
WHERE IS MY DATA LOCATED?
LSR.signage uses the existing data from your database. This means that there is no second data set that you need to back up.
HOW SAFE IS MY DATA?
Your data sources are qualified and validated via dedicated LSR.signage servers. These are protected against unauthorized access by the highest security features.
HOW DO I MANAGE THE SYSTEM?
Our HMI systems run twenty-four-seven. Administration is done in no time at all using the LSR.dash administration software, which is included in the LSR.signage package. This allows you to control all MODI and other connected displays effortlessly and clearly.
CAN THE SYSTEM BE VALIDATED?
Certainty! With Life Science Ready, Systec & Solutions stands for the GMP-compliant running of your production in the clean room. By validating your data, production processes and ultimately the health of patients and consumers are protected. LSR.signage ensures that all automation processes meet your requirements and potential risks are prevented.
IS THE SYSTEM AFFORDABLE?
Yes, because the solution consisting of LSR.signage is pricelessly efficient. Implementation is simple and saves time and money. You also benefit from our extremely fair and transparent pricing. This includes optional customization options.
Need assistance?
Do you have any questions about our products or would you like some assistance? Contact us now and we will be happy to help you find the optimum solution for your requirements without obligation. Fill out our contact form or give us a call on +49 721 66351 0.
